True Health Online

View more
View moreWhat's the difference between healthcare and Medicare?
Alright folks, let's dive into this pool of healthcare lingo but don't worry, I won't let you drown! First off, healthcare is like that big umbrella that keeps you dry when it's pouring medical terms, bills, and services. Now, under that healthcare umbrella, you've got Medicare, a cheeky little government program that helps people over 65, or those with certain disabilities, not to get soaked by medical costs. So, in the rainstorm of life, healthcare is the umbrella itself while Medicare is like the handy button that pops the umbrella open when you need it. Remember, there's no need to swim in confusion, just keep floating with me!
View more
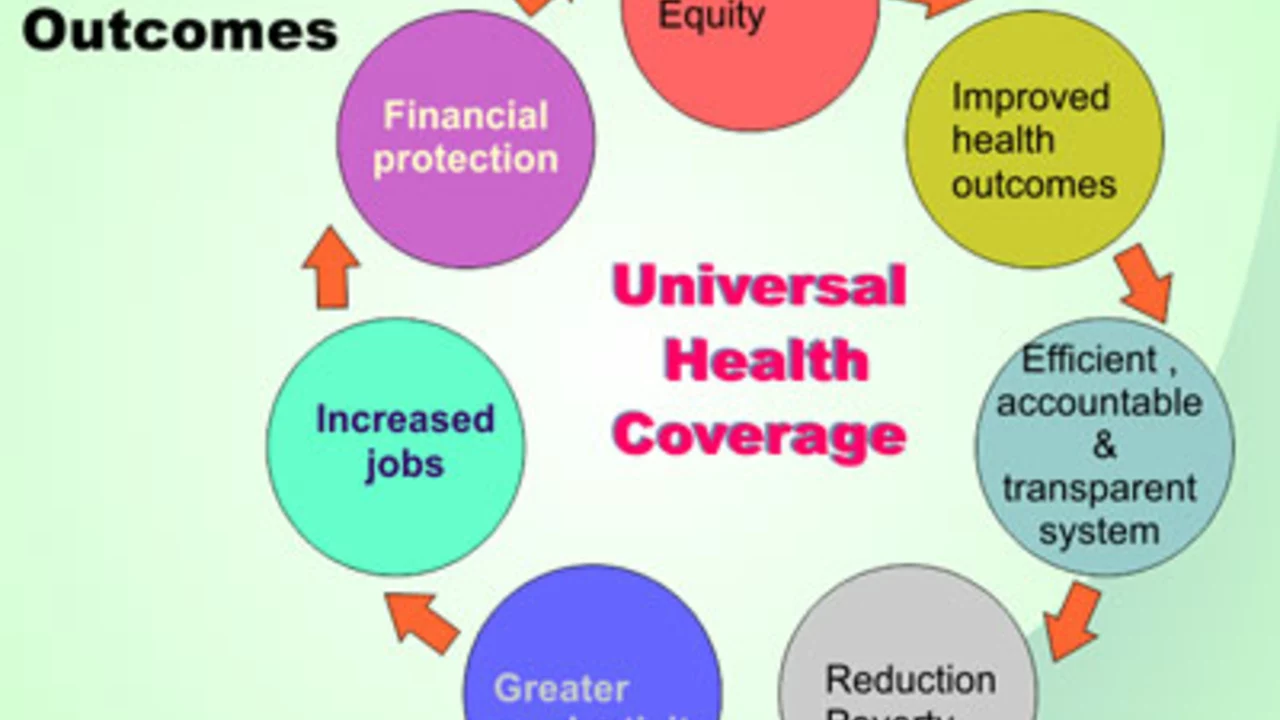
View moreWhy is universal health care a bad idea?
Buckle up folks, because we're diving into the wacky world of universal healthcare, a concept as confusing as my grandmother's remote control! First off, it's like a magic trick with tax money - poof! Higher taxes everywhere to cover costs. Then there's the issue of waiting times, where getting an appointment could take longer than reading the entire 'Game of Thrones' series. Plus, the quality of care could drop faster than a hot potato, with doctors being paid less and working more. Lastly, it takes away our freedom to choose our healthcare, placing it in the hands of bureaucrats who probably can't even choose their lunch without a committee!

View more
View moreWhy is healthcare tied to employment in the US?
In the US, healthcare is intricately linked to employment, a model that dates back to World War II when wage freezes led companies to offer health insurance as a benefit to attract workers. This system, unique to the US, has continued due to tax incentives and the Employee Retirement Income Security Act (ERISA) of 1974 that encourages businesses to provide health benefits. Unfortunately, this model leaves those without employment often without healthcare. As a result, millions struggle to access affordable healthcare on their own, causing a significant issue that the country continues to grapple with today. So, while employer-based health insurance has its roots in historical circumstances, its persistence is largely due to legislative choices and tax policies.

View more
View moreWhat role should governments play in ensuring public health?
In tackling public health issues, governments play a crucial role. They're responsible for creating and implementing policies that promote health and well-being, such as regulations on food safety and pollution. They also provide funding for necessary health services and research. As a key player, the government must ensure access to health care for all citizens, regardless of their income. It's their duty to protect us from health risks and help us maintain a healthy lifestyle.

View more
View moreWhat is the health care system like in Mexico?
As a blogger, I've been researching the health care system in Mexico and found that it's quite different from what I'm used to. The country has a mix of public and private health care, with the public sector being divided into two main systems: Seguro Popular and IMSS. While Seguro Popular provides basic medical coverage to millions of uninsured Mexicans, IMSS is a more comprehensive health care plan for those with formal employment. On the other hand, the private health care sector offers more personalized and faster services, but at a higher cost. Overall, the Mexican health care system is quite diverse, with options to cater to different needs and financial situations.